Molecular Geometry of XeF4 [with video and free study guide]
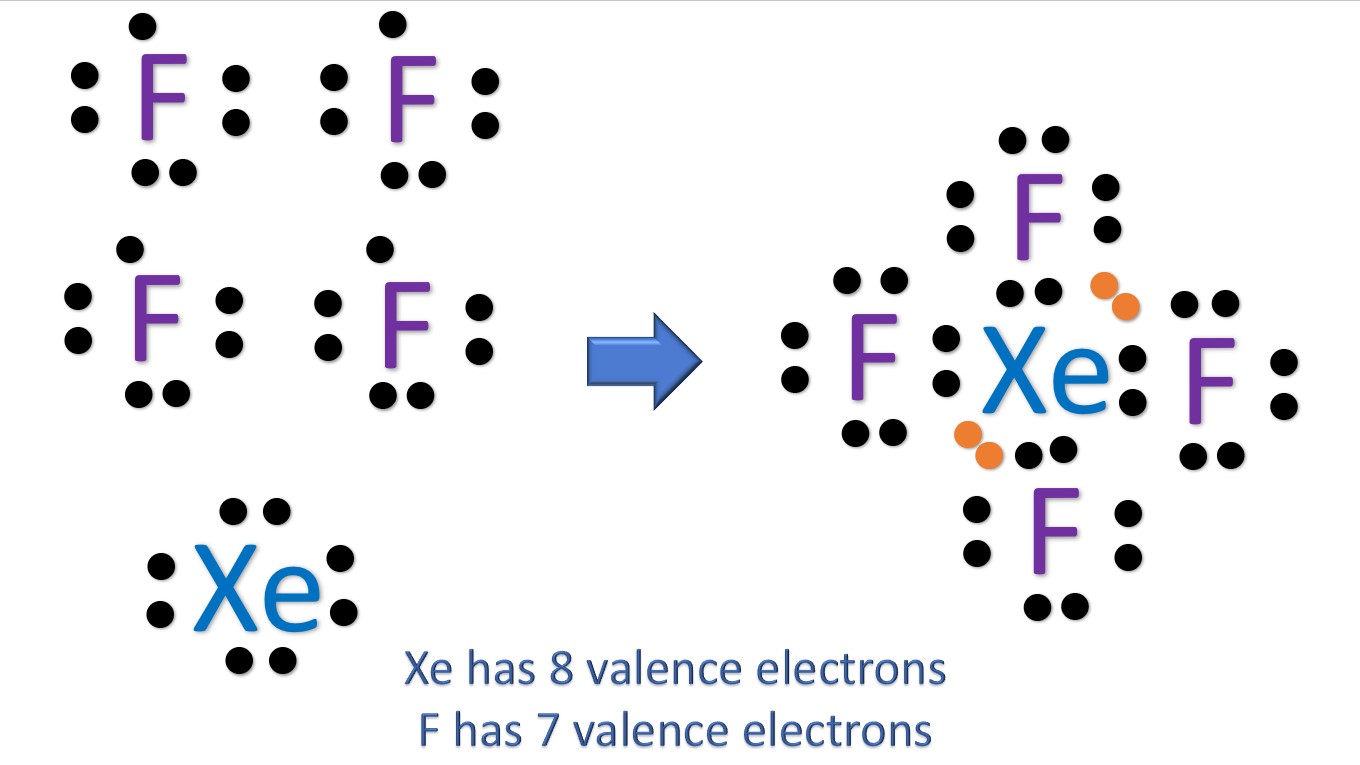
The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe.. Let's do the XeF4 Lewis structure. Xenon has 8 valence electrons. Fluorine has 7, but we have four of the Fluorines; so that gives us 8 plus 28: 36 valence electrons. We'll put Xenon in the center, it's the least electronegative; and then Fluorines on.
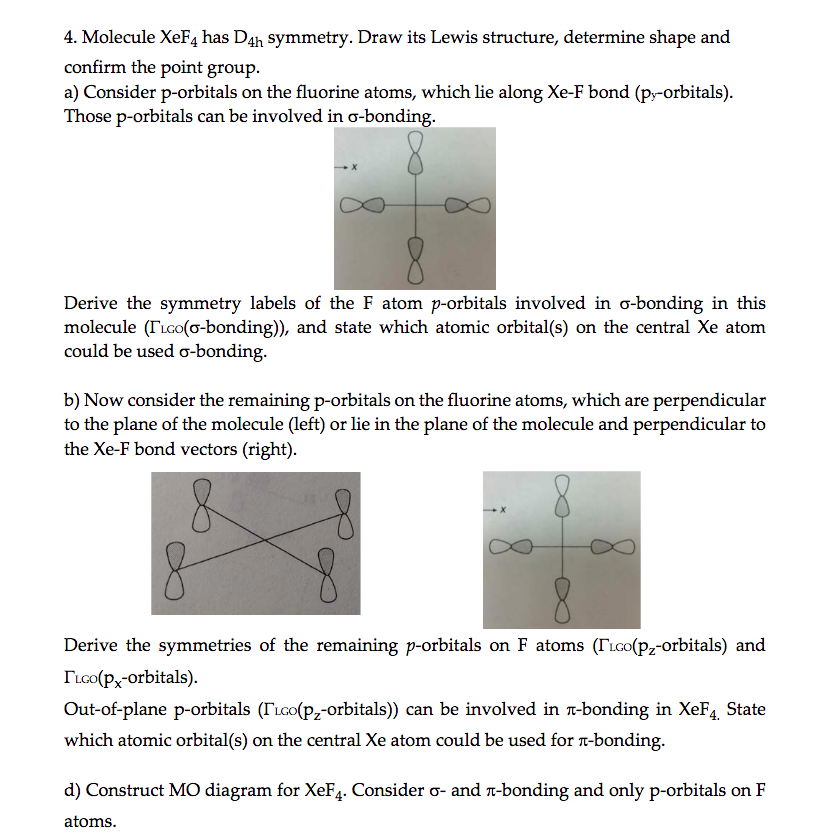
Solved 4. Molecule XeF4 has Dsh symmetry. Draw its Lewis
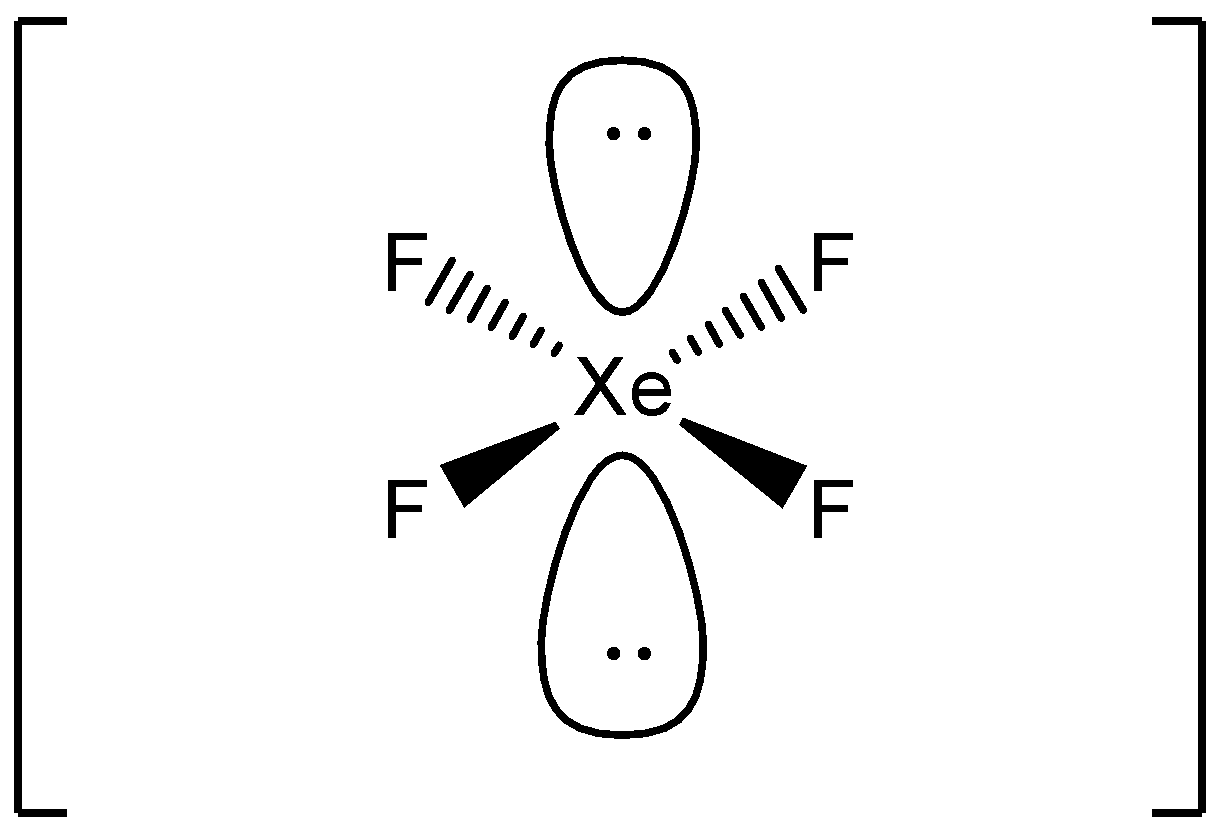
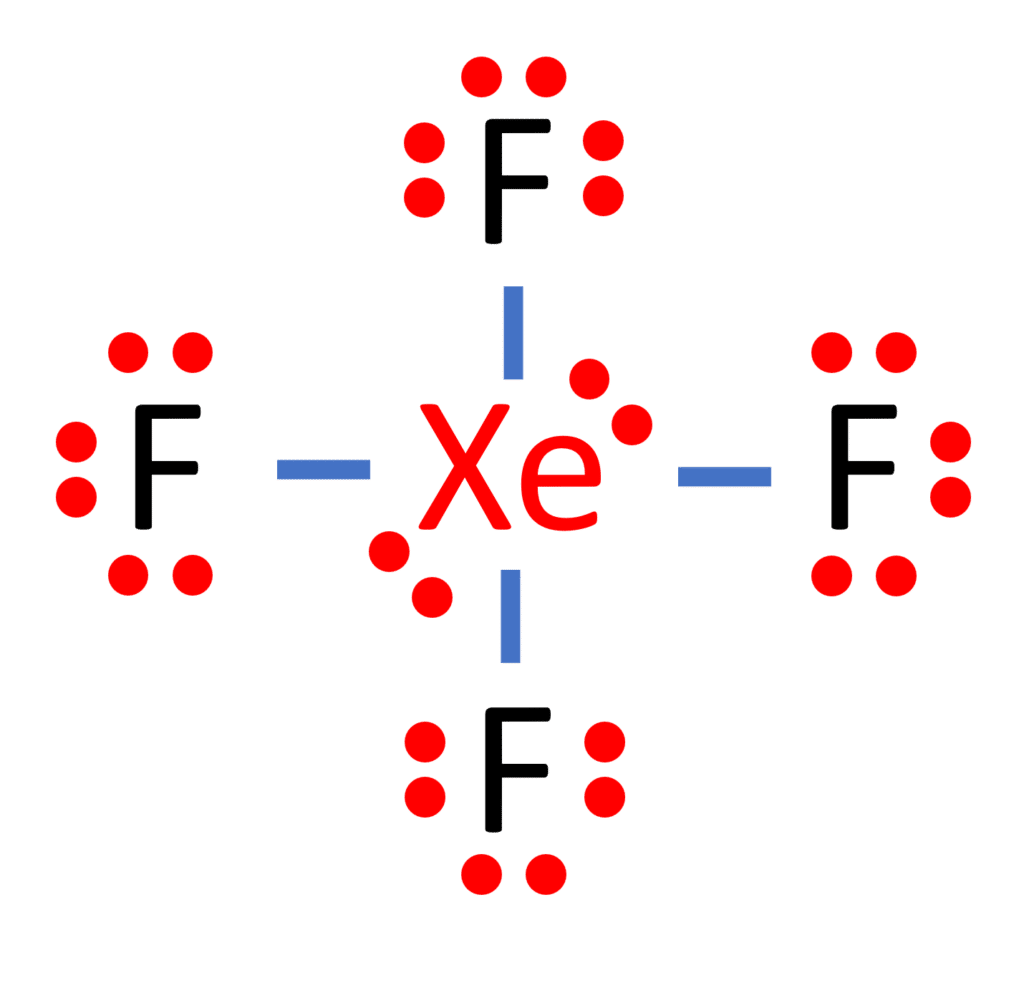
Lewis structure of XeF4 contains four single bonds between the Xenon (Xe) atom and each Fluorine (F) atom. The Xenon atom (Xe) is at the center and it is surrounded by 4 Fluorine atoms (F). The Xenon atom has 2 lone pairs and all the Fluorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.

So far, we’ve used eight of the XeF4 Lewis structure’s total 8
328 39K views 3 years ago An explanation of the molecular geometry for the XeF4 (Xenon tetrafluroide) including a description of the XeF4 bond angles. The electron geometry for the Xenon.
XeF4 Molecular Geometry, Bond Angles & Electron Geometry YouTube
XeF4 lewis structure involves one atom of xenon and four fluorine atoms. Xenon (Atomic number = 54 and electronic configuration = 2,8,18,18,8) belongs to group 18 of the periodic table and has 8 valence electrons.

Is XeF4 Polar or Nonpolar? Techiescientist
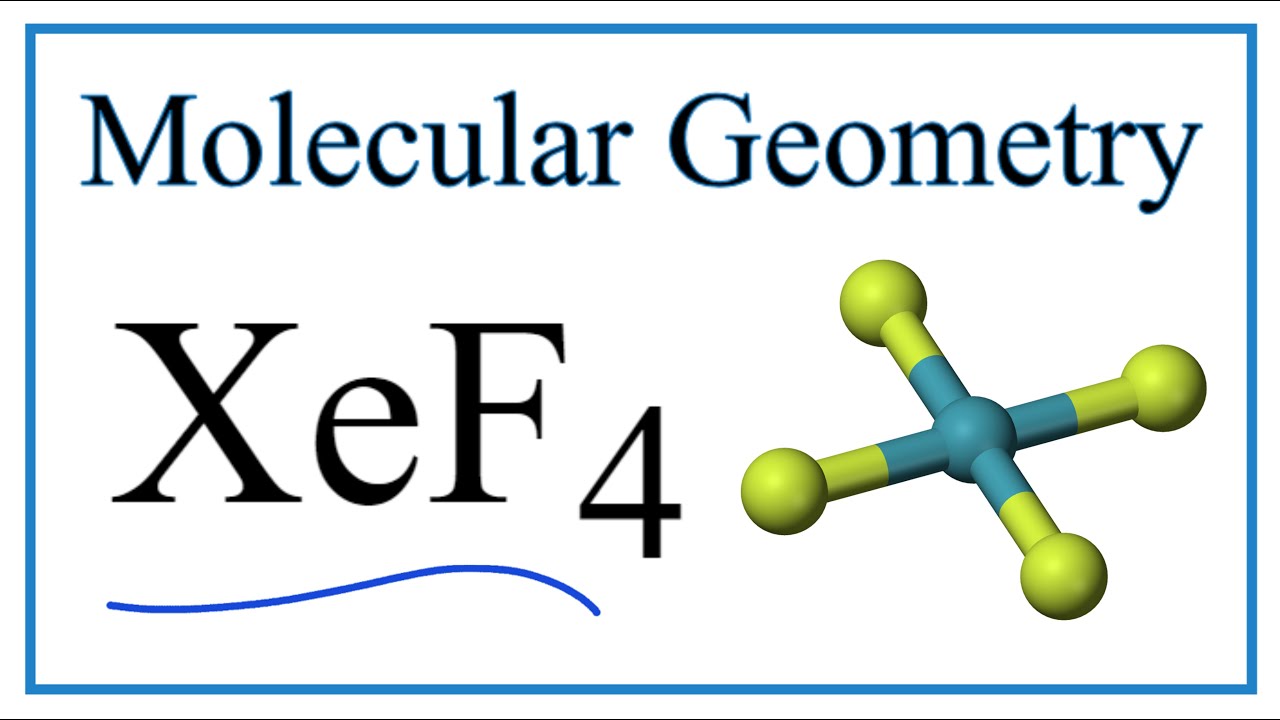
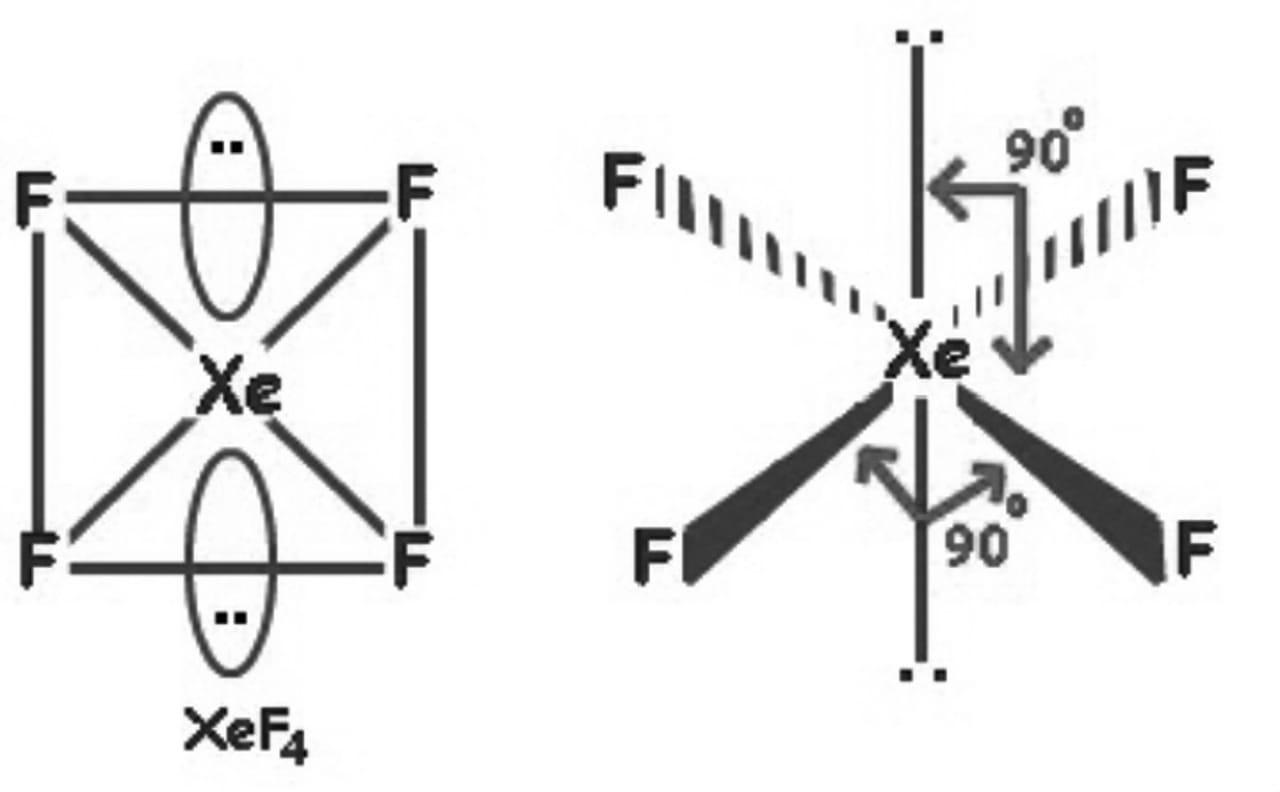
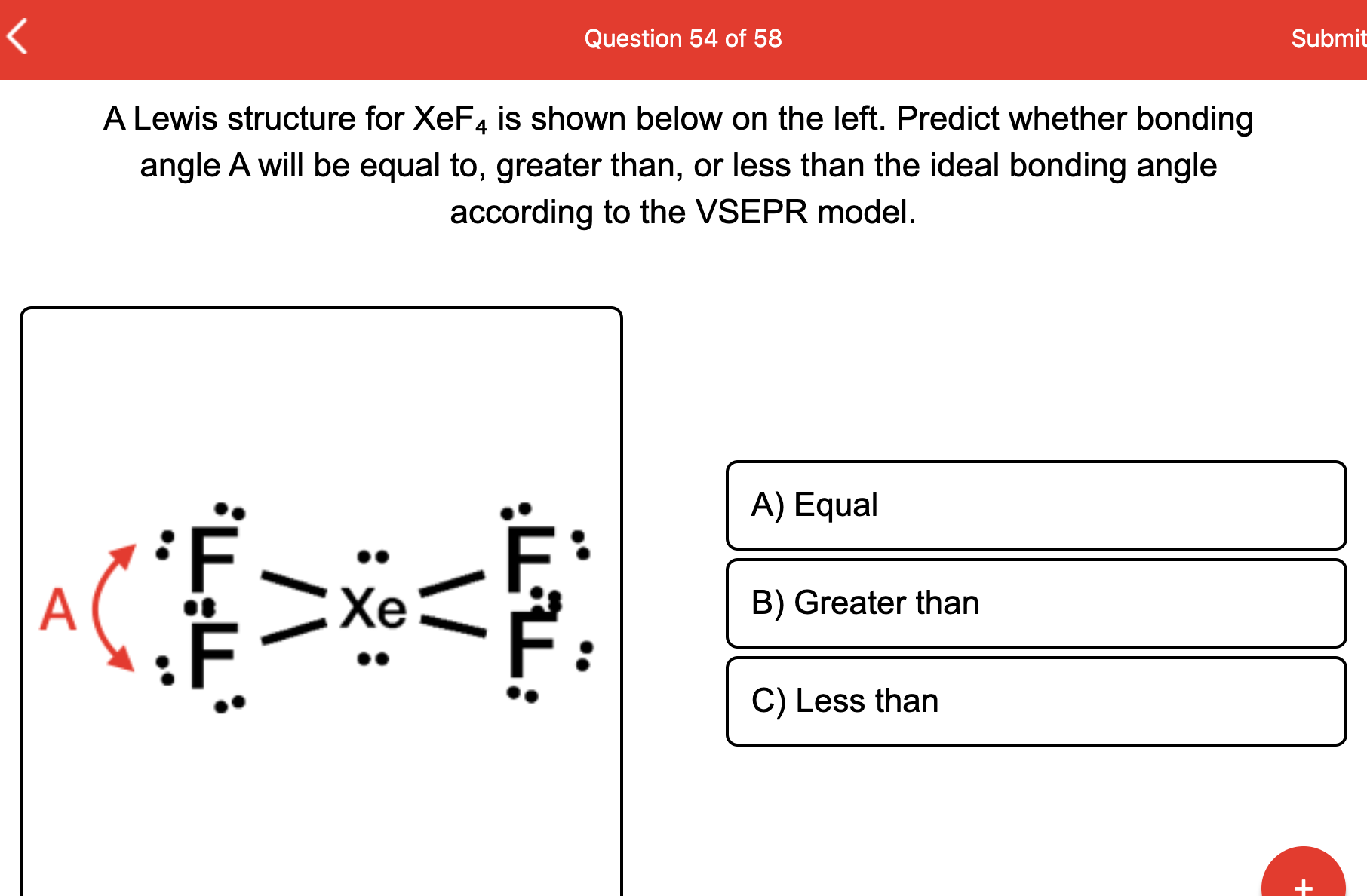
Its structure was determined by both NMR spectroscopy and X-ray crystallography in 1963. [6] [7] The structure is square planar, as has been confirmed by neutron diffraction studies. [8] According to VSEPR theory, in addition to four fluoride ligands, the xenon center has two lone pairs of electrons. These lone pairs are mutually trans. Synthesis

Hello Guys! Today we are going to look at the Lewis Structure of XeF4
For making the Lewis structure, we need to know the valence electrons of XeF4 to make its structure and know the placement of atoms in the molecule. Contents XeF4 Valence electrons XeF4 Lewis Structure XeF4 Hybridization XeF4 Molecular Geometry XeF4 Bond angles XeF4 Polarity - Is XeF4 Polar or Nonpolar? XeF4 Valence electrons
Molecular Geometry Of Xef4 Youtube
1 Answer Stefan V. Aug 4, 2016 See explanation. Explanation: The first thing to do here is calculate how many valence electrons you have in one molecule of xenon tetrafluoride, XeF4. To do that, add the number of valence electrons that each atom brings to the table. You will have Xe: 8 e− aF: 7 e−
Xef4 Vsepr
The first step is to sketch the Lewis structure of the XeF4 molecule, to add valence electrons around the xenon atom; the second step is to add valence electrons to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the XeF4 Lewis Structure.

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube
XeF4 is the chemical formula of the compound Xenon Tetrafluoride. This chemical compound is formed when xenon reacts with fluorine. Its chemical equation could simply be written as : Xe + 2F2 ——> XeF4 In this process, elemental fluorine supposedly oxidizes xenon, under some specific conditions of temperature and pressure.

Solved The Lewis structure for XeF4 is Xě Which of
Xenon tetrafluoride (XeF4) is a square planar, non-polar molecule. The Xenon atom has 4 bonding pairs of electrons and 2 lone (non-bonding) pairs of electro.
XeF4 Lewis Structure 4 Simple Steps What's Insight
Science Chemistry Chemistry questions and answers Write the Lewis structure for XeF4. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Include all lone pairs of electrons. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Solved
In the Lewis structure of XeF4 structure there are a total of 36 valence electrons. XeF4 is also called Xeon Tetrafluoride. Note that XeF4 can have an Expanded Octet and have more than.

Solved Molecule XeF4 has D4h symmetry. Draw its Lewis
Hello Guys!Today we are going to look at the Lewis Structure of XeF4 ( Xenon Tetrafluoride )Although Xenon is a noble gas it reacts with four Fluorine atoms.

XeF4 Lewis structure, Molecular geometry, Bond angle, Shape
For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. The Lewis structure for XeF4 has a total of 36 valence electrons.

XeF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
XeF4 lewis structure has Xenon atom (Xe) at the center which is surrounded by four Fluorine atoms (F). There are 4 single bonds between the Xenon atom (Xe) and each Fluorine atom (F). There are 2 lone pairs on the Xenon atom (Xe) and 3 lone pairs on all the four Fluorine atoms (F).
[Solved] Complete the chart. Molecular Polarity. XeF4 +Lewis Structure
XeF4 Lewis Structure, Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.